““The successful achievement of the Directorate General of Pharmacy's programs and activities in achieving the target performance indicators in the first year of the 2020-2024 Strategic Plan is the result of the hard work of all components, optimal utilization of resources and strengthening central and regional coordination, especially in program and activity planning, preparation of legislation in the field of pharmacy and medical devices and continuous monitoring and evaluation of the implementation of activities.”“
Delivered by Acting. Director General of Pharmaceuticals - drg. Arianti Anaya, MKM at the opening of the Eastern Regional RAKONAS Farmalkes (27/04/2021).
In her remarks as well as the Progress Report on Program Implementation at the Directorate General of Pharmaceuticals and Medical Devices until 2020, the Priority Program for 2021, the implementation of programs that support access - support for primary care, pharmaceutical and medical device resilience and quality assurance of medical device products (pre- and post-market).
This RAKONAS activity was held to increase understanding and synergy between the central and regional levels, in the implementation of programs and activities of the Directorate General of Pharmacology, to provide support for provinces / regencies / cities that have not yet reached the target with better efforts and cooperation to achieve Strategic Goals, Program / Activity Objectives and Program / Activity Performance Indicators in the Ministry of Health Strategic Plan 2020-2024.
Five Strategic Objectives with eight Strategic Targets in Kepmenkes Number 21 of 2020 concerning the Ministry of Health's Strategic Plan 2020-2024, where one of the targets is to increase access, independence and quality of pharmaceuticals and medical devices in order to achieve the goal of improving health resources. In 2020, the achievement of this target is measured through the indicator of the percentage of health centers with the availability of essential medicines with a target of 85%. The achievement in 2021 was 92.12% or with a percentage achievement of 108.38%.
Health Service Support Program and National Health Insurance (JKN) with the target of increasing access, independence and quality of pharmaceutical preparations and medical devices with five achievement indicators in 2020, among others: Percentage of regencies/cities with the availability of essential drugs by 77%; Percentage of qualified medical devices by 91%; Percentage of Puskesmas with the availability of IDL vaccines (Complete Basic Immunization) by 90%; Percentage of types of pharmaceutical raw materials that can be produced domestically by 15%; and Percentage of medical devices that can be produced domestically by 55%.
From the 2020 performance indicators, it has reached the set targets, namely for the realization of the percentage of regencies / cities with the availability of essential drugs of 83.75% or with a percentage achievement of 108.77%; Realization of the percentage of medical devices that meet the requirements of 92.70% or with a percentage achievement of 101.87%; Realization of the percentage of Puskesmas with the availability of IDL vaccines (Complete Basic Immunization) of 96.98% or with a percentage achievement of 107.76%; Realization of the percentage of types of pharmaceutical raw materials that can be produced domestically of 16.67% or with a percentage achievement of 111.11%; and Realization of the percentage of medical devices that can be produced domestically of 55.56% or with a percentage achievement of 101.02%.
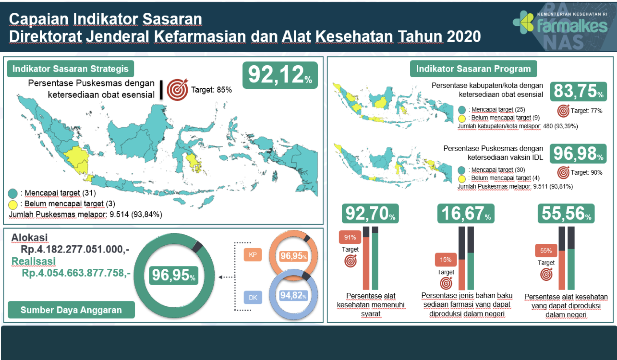
The implementation of the 2020 program is supported by budget resources sourced from the Central Office and Deconcentration with an allocation of 4.18 trillion rupiah with a realization of 4.05 trillion (96.95%).
The allocation of DAK Fisik Subfield of Pharmaceutical Services in 2020 amounted to 1.366 trillion rupiah with a total of 519 prov / regency / city recipients with details of 92% allocation for the provision of drugs and BMHP in regencies / cities with a total of 496 proposed packages; 5% allocation for the provision of prov / regency / city pharmaceutical installation infrastructure with a total of 5,818 proposed units; and 3% allocation for new construction or rehabilitation of prov / regency / city pharmaceutical installations with a total of 32 proposed work packages. Based on data from DJPK and the SIMADA application, the realization of the absorption of DAK Fisik Subfield of Pharmaceutical Services in 2020 was 85.80% or a total of 1.1 trillion rupiah.
In handling the Covid-19 pandemic while increasing national health resilience, synergy with stakeholders resulted in an increase in the production capacity of domestic medical devices in the production of ventilators, High Flow Nasal Canule (HFNC), rapid antigen tests, Real Time Reagent - Polymerase Chain Reaction (RT-PCR) / RT-Lamp, Oxygen Helmet, and N95 masks.
The 2021 Priority Program is prepared with a focus on improving access, independence, and quality of pharmaceutical preparations and medical devices. In the aspect of access, the root problems that need intervention are optimizing the management of the logistics management cycle/supply chain of drugs and vaccines; In the aspect of independence, optimizing domestic production for medical devices and pharmaceutical raw materials, optimizing product use, and constraints on the limited choice of halal-certified vaccines; In the aspect of quality, consistency in meeting standards in pre-market supervision and post-market supervision needs intervention.
National Digital Inventory (DIN) is a form of drug supply chain management intervention, in realizing effective and efficient drug management. This system is single entry, digital, real time, user friendly and connected to other applications, such as SIKOBAT, E-logistics, PBF E-report, and e-monv. This DIN will be integrated and developed gradually to be utilized by Provincial / Regency / City Health Office, pharmaceutical industry and distributors, as well as Health Facilities and Work Units throughout Indonesia, so that it is expected that in addition to integration, it can also assist data management and decision making in every logistics management process and create better control of the national drug and BMHP supply chain.
The government is in the process of securing the vaccine doses needed to meet the national vaccination plan targets. The government has established a vaccination supply plan in line with the vaccination rate required to achieve herd immunity by 2021. It is expected that by regularly evaluating to mitigate risks such as bulk supply, production capacity, and export-import policies, up to 60 million vaccine doses per month can be achieved, to ensure the availability of vaccines for the community and achieve herd immunity as soon as possible.
In order to accelerate vaccination, vaccine distribution is arranged to go directly to regencies/cities. This distribution plan is strengthened by the implementation of a vaccine distribution management system, which utilizes digital technologies such as the Internet of Things (IoT), Track and Trace, Transport Management System, and an integrated BI Dashboard. This system can be monitored through the Command Center as it is currently operating, making it easier to monitor vaccine distribution in real time.
The Provincial Health Office, and Regency/City Health Office, will be actively involved in this system, along with the vaccine distributor appointed by the Ministry of Health (PT Bio Farma), as a form of synergy between all components of the nation. Currently, the Ministry of Health is in the process of building a vaccination dashboard that can be seen by the general public. The dashboard will contain data on distribution and use down to the district/city level, thus providing control and feedback on the implementation of this vaccination.
In terms of realizing self-reliance, a comprehensive approach must be taken. Efforts to build independence are multi-sector, capital-intensive, and innovation-intensive. For this reason, as a national guide, Presidential Instruction No. 6/2016 is available, which serves as the basis for regulation of multi-sector collaboration to accelerate the development of the pharmaceutical and medical device industries.
In the health sector, an Action Plan for the Acceleration of the Development of the Pharmaceutical and Medical Device Industry has been developed, through Permenkes 17/2017. The plan has mapped out the targets to be achieved in the period up to 2025, in realizing pharmaceutical and medical device independence.
Optimizing the role of the Provincial and Regency / City Health Office in pharmaceutical and medical device programs, among others: Optimizing logistics management of drugs and BMHP so as to increase and ensure availability at the Puskesmas level and ensure drug quality; Optimizing the use of DAK to support efforts to achieve targets in the field of pharmaceuticals and medical devices; Taking an active role in the implementation of DIN to support the integration of drug distribution and availability data in real time; Increasing the role of health offices in post market supervision of medical devices and PKRT; Optimizing the management of Covid-19 logistics at both the provincial and regency/city levels to vaccination facilities; Improving logistics management of vaccines and their supporters for vaccination; Strengthening monitoring of the availability of essential drugs at Puskesmas and improving the accuracy of RKO; Coordinating with the center on program technical implementation and managerial support.
**humas_farmalkes2021



 Acting. Director General of Pharmaceuticals - drg. Arianti Anaya, MKM at the opening of the Eastern Regional RAKONAS Farmalkes, April 27, 2021
Acting. Director General of Pharmaceuticals - drg. Arianti Anaya, MKM at the opening of the Eastern Regional RAKONAS Farmalkes, April 27, 2021















