In early 2020, the world was suddenly disrupted by a pandemic caused by a novel strain of covonaviruses (CoV), SARS-CoV-2. The disease caused by SARS-CoV-2 infection is then referred to as coronavirus disease or COVID-19. In Indonesia, the first case of COVID-19 was found in early March 2020.
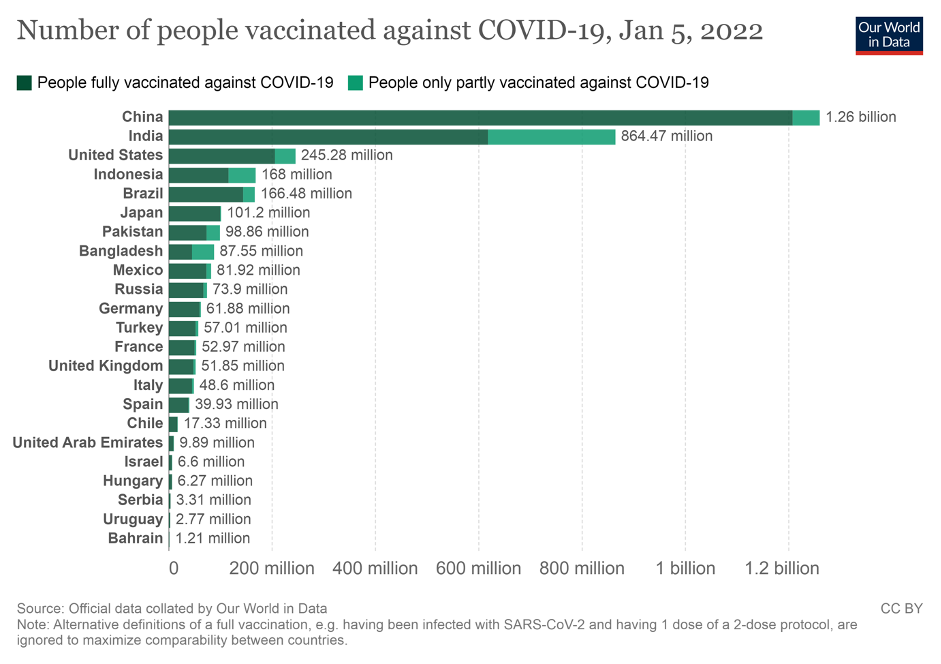
Various strategies have been implemented by the Indonesian government to overcome the impact of the pandemic both through economic recovery strategies and increasing the capacity of the health system. One of them is through the provision of vaccines and the implementation of COVID-19 vaccination. The provision of this vaccine is a form of support and commitment to the implementation of COVID-19 vaccination to a target of 208 million Indonesians in order to achieve community immunity and pandemic containment. For these efforts, Indonesia is currently listed as the fourth largest country in the world based on the number of people who have been vaccinated and based on total injections in the world.
The COVID-19 vaccine arrived in Indonesia for the first time on December 6, 2020 totaling 1.2 million doses under the CoronaVac vaccine brand made by Sinovac. The COVID-19 vaccination program in Indonesia began by the government on January 13, 2021 with the first vaccine recipient in Indonesia, President Joko Widodo. The COVID-19 vaccine used is of course after obtaining an Emergency Use Authorization (EUA) from the Food and Drug Administration (BPOM).
The supply of COVID-19 vaccines in Indonesia is obtained through bilateral mechanisms (APBN), COVAX grants or with grants from other countries. The number of vaccines imported in Q1-Q2 2021 amounted to 69.5 million doses by bringing in CoronaVac and AstraZeneca finished vaccines and bulk from Sinovac which continued production by PT Bio Farma (Persero). The first semester of 2021 is the most difficult period in obtaining vaccine supplies where not all types of vaccines have obtained EUA licenses from BPOM and the high demand for the need to obtain vaccines between countries. For this reason, the government is trying to improve bilateral and multilateral coordination to obtain a better vaccine supply.
In Q3 2021, vaccine supply began to increase where the number of vaccines obtained reached 157.6 million doses and more types of vaccines were imported such as Moderna, Sinopharm, Pfizer and Janssen. In Q4 2021, the total supply reached 201.9 million doses and more vaccine types were imported such as Novavax. December was recorded as the month with the highest supply throughout 2021 with a realized supply of 82.7 million doses.
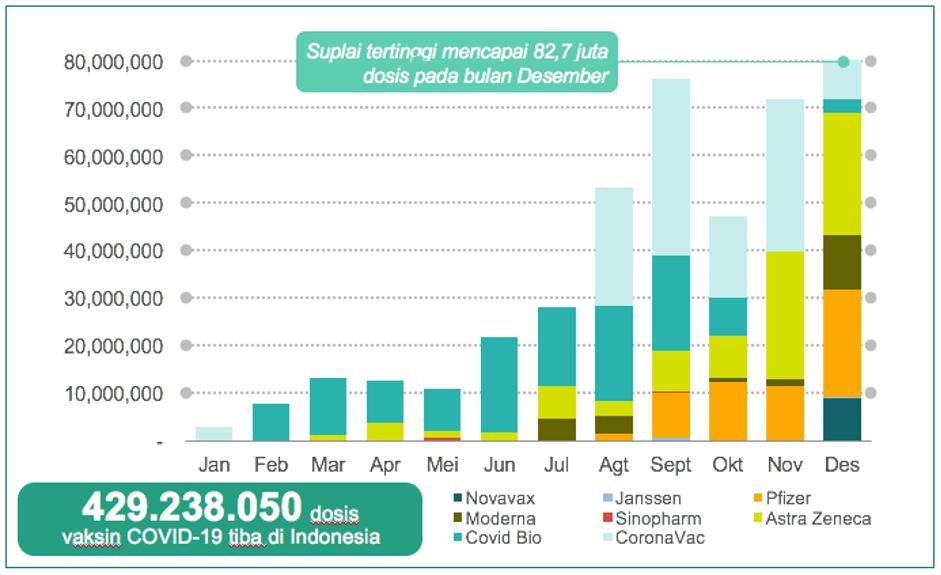
As of December 31, 2021, eight vaccine brands have been received by Indonesia totaling 429.2 million doses consisting of CoronaVac, Covid Bio, Astra Zeneca, Sinopharm, Moderna, Pfizer, Janssen and Novavax and vaccination coverage has reached 79.4% for the first dose and 54.68% for the second dose. This has exceeded the WHO target of 40% of the population being fully vaccinated by the end of 2021.

The surge in cases that occurred in almost all countries in the world resulted in scarcity of vaccine stocks, including vaccine stocks in Indonesia. Therefore, the government continues to strive to overcome the scarcity of COVID-19 vaccine stocks, one of which is to collaborate with various countries to meet the needs of the COVID-19 vaccine in Indonesia.
Eight countries have bilaterally provided COVID-19 vaccine grants to Indonesia, including: UAE, Japan, Australia, Netherlands, China (Government of China, Red Cross, Sinovac), Singapore, France, and UK. In addition to bilateral sources, Indonesia also obtained multilateral grants through COVAX. Several countries that donated COVID-19 vaccines through Multilateral grants include: America, Germany, France, the Netherlands, Italy, New Zealand, Ireland, Spain, and the European Union.
Vaccines that arrive in Indonesia need to go through several stages until they can be distributed to the regions. The vaccines are first stored in a storage room at PT Bio Farma (Persero) and are systematically recorded both in number and type in the SMDV (Vaccine Distribution Management System). Before being released and ready for distribution, the vaccines will go through an inspection and sampling process by BPOM to ensure safety, efficacy and quality. Then the Ministry of Health determines the allocation of the number of vaccines for each province and city district based on the need and speed of vaccination. and issues a distribution order to PT Bio Farma (Persero) to carry out the distribution.
In order to achieve optimal distribution capacity to all parts of Indonesia, the Ministry of Health involves state-owned and private vaccine distributors. The distribution plan that has been prepared will be strengthened by implementing a vaccine distribution management system. This system utilizes digital technologies such as the Internet of Things (IoT), Track and Trace, Transport Management System, and BI Dashboard which are gradually integrated with other systems inside and outside the vaccine distributor. This system can be monitored through the Command Center as it is currently operating, making it easier to monitor vaccine distribution in real time.
Provincial Health Offices, and District/City Health Offices, will be actively involved in this system, along with the vaccine distributor appointed by the Ministry of Health (PT. Bio Farma). This involvement is a form of synergy between all of us in order to succeed the implementation of vaccination. In addition to vaccines, the Ministry of Health will also distribute vaccination support logistics (ADS, alcohol swabs, and safety boxes) according to the delivery of vaccine doses to the Provinces.Throughout 2021, a total of 390,540,236 vaccine doses have been released and a total of 335,909,618 doses or around 86% of vaccine doses have been distributed to all regions of Indonesia.

At the beginning of the 2021 fiscal year, the budget requirement to achieve the need for COVID-19 vaccine doses in Indonesia reached 50.2 trillion rupiah. It is planned that with this budget amount, Indonesia can obtain a total of 423.8 million doses of COVID-19 vaccine as an effort to deal with the COVID-19 pandemic. During the implementation of vaccine supply activities in 2021, efforts to provide the number of vaccine doses were taken with several other mechanisms such as increasing the optimization of multilateral relations (COVAX grants), optimizing relations between countries (bilateral grants), and implementing a mutual cooperation vaccine program. So that at the end of the year, the Government can provide 422.9 million doses of vaccine by saving a budget of up to 16.1 trillion ruliah from the initial 2021 budget requirement allocation. The successful implementation of vaccination starting from the target health workers, the elderly, vulnerable communities and children cannot be separated from the support of all parties as an effort to limit the COVID-19 pandemic. However, in addition to carrying out vaccinations, we must still comply with the 5M health protocol, namely by wearing a mask, maintaining distance, washing hands using soap and running water, staying away from crowds, and limiting mobility, so that it is more effective in reducing the rate of spread of the COVID-19 virus.



 illustration: vaccine
illustration: vaccine















