Jakarta, April 14, 2023, The government makes it easy for the public to test for COVID-19 independently using antigen rapid tests. Currently, there are self-antigen rapid test products available that have obtained distribution permits from the Ministry of Health, with a reporting system through the SATUSEHAT application.
Director General of Pharmaceuticals and Medical Devices, Dr. L. Rizka Andalucia, hopes that the self-test will accelerate early detection and treatment efforts for COVID-19. Given that there is currently an increase in COVID-19 cases along with the discovery of two cases of the XBB.1.16 variant or better known as Arcturus in Indonesia.
"Self-screening is expected to accelerate the finding of COVID-19 cases and treatment. It is hoped that independent screening in the context of early detection of COVID-19 can be carried out well for a healthier and more resilient Indonesia," said Director General Rizka.

The self-antigen rapid test is an independent COVID-19 test method, which in the process does not require the assistance of health workers, both during specimen collection, to reading test results, as an independent screening effort in the context of early detection of COVID-19.
The public can buy self-antigen rapid test products at medical device stores, pharmacies, and other places that have a license to distribute medical devices. Make sure the purchased self-antigen rapid test product has a distribution license. Currently, there are two (2) self-antigen rapid test products that have been approved for distribution and have Quick Response (QR) codes linked to the SATUSEHAT application, namely FASTCLEAR Q COVID-19 Ag Nasal and Panbio COVID-19 Antigen Self-Test.
If there are other self-antigen rapid test products that have also obtained distribution authorization from the Ministry of Health, the public can access the information through https://infoalkes.kemkes.go.id. The product has a Quick Response (QR) code that is linked to the SATUSEHAT application. The QR code contains a unique code for each product, as a product identifier so that it is traceable and cannot be reused.
When confirmed positive for COVID-19, the Government also provides telemedicine services for people with positive COVID-19 test results. Therefore, the public should not hesitate to report the results of the self-antigen rapid test through the SATUSEHAT application, upload the test results by scanning the QR code and filling in the required data.
"If the test result is positive, then carry out self-isolation and continue consultation through telemedicine services to get free treatment. If the test result is negative, but symptomatic or in close contact, then continue to self-quarantine and consult with health workers," continued Director General Rizka.

The things that people need to pay attention to are:
- Use a licensed self-antigen test product.
- Test results can be inputted through the SATUSEHAT application in the COVID-19 Test Results section.
- To obtain valid results, follow the product usage instructions on the packaging.
- Pay attention to the waste disposal method of the COVID-19 antigen rapid test that has been used, namely:
- After use, the swab kit is put into a plastic bag and then disinfected with disinfectant by spraying.
- The plastic bag used must be strong/leakproof.
- It is then combined with similar waste (plastic) and should not be mixed/included with organic waste (wet waste).
- Direct disposal into landfills is prohibited.
Indonesia has moved towards the transition from pandemic to endemic. This does not mean we are free from the COVID-19 virus, as seen from the increase in cases since March 2023. However, with the learning and joint efforts during these 3 years, we are now ready and have complete modalities, including the availability of domestic vaccines, the availability of therapeutic drugs, and the availability of COVID-19 diagnostic medical devices.
The latest oral antiviral drug called nirmatrelvir/ritonavir or Paxlovid is a new COVID-19 drug that is now available in Indonesia. This drug is considered more effective in the healing process of COVID-19 patients, which is given to adult patients with mild to moderate severity and the potential to become severe.
A total of 24,096 doses were donated to Indonesia by the Governments of the United States and Australia. Paxlovid will be distributed to 34 provinces, with the initial phase of drug distribution prioritized to areas in dire need.
This news was broadcast by the Bureau of Communication and Public Services, Ministry of Health RI. For further information, please contact the Ministry's Halo hotline number 1500-567, SMS 081281562620 and email [email protected] (NI).
Head of Bureau of Communication and Public Service
dr. Siti Nadia Tarmizi, M.Epid



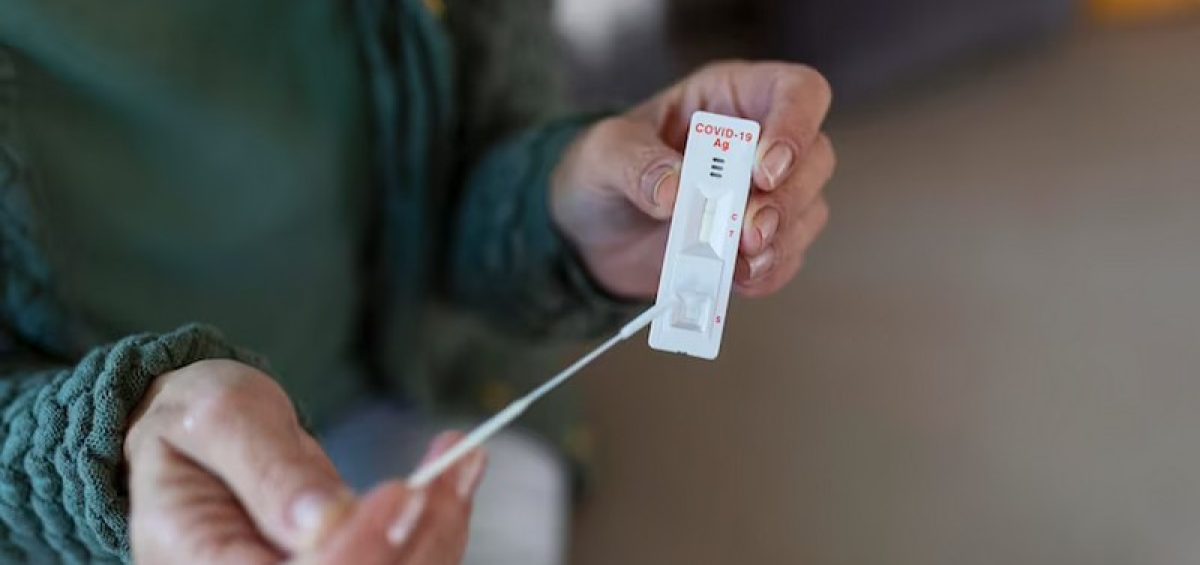 Illustration: Covid-19 antigen rapid test
Illustration: Covid-19 antigen rapid test















