Jakarta, 21 September 2023.
On the third day of the Public Hearing on the Draft Derivative Regulation of Health Law No. 17 of 2023, the Directorate General of Pharmaceuticals and Medical Devices discussed the topic of Safeguarding Pharmaceutical Supplies, Medical Devices and Household Health Supplies (PKRT).
This activity invited stakeholders from various sectors, including BPOM, BPJPH, Ministries / Institutions, Professional Associations and Organizations, Communities and Foundations, BSN, BNN, BAPETEN, Health Services, Expert Practitioners.
Secretary of the Directorate General of Pharmaceuticals and Medical Devices, Dita Novianti at the opening said that after the enactment of the Health Law, it received a lot of attention from the community, because this law is the first law to be compiled in an omnibus law, where the contents are comprehensive related to health. Not everything is regulated in the Health law, so it will be followed up with the preparation of implementing regulations. There are 101 articles delegated in the RPP which is a derivative of the Health Law, 2 articles delegated in the draft Presidential Regulation and 5 Articles delegated in the draft Minister of Health Regulation.
"The purpose of this public participation is to accommodate input from the community, we hope that associations, professional organizations representing organizations help channel the aspirations of members for us to jointly provide constructive input on the RPP that is being made," said Dita.

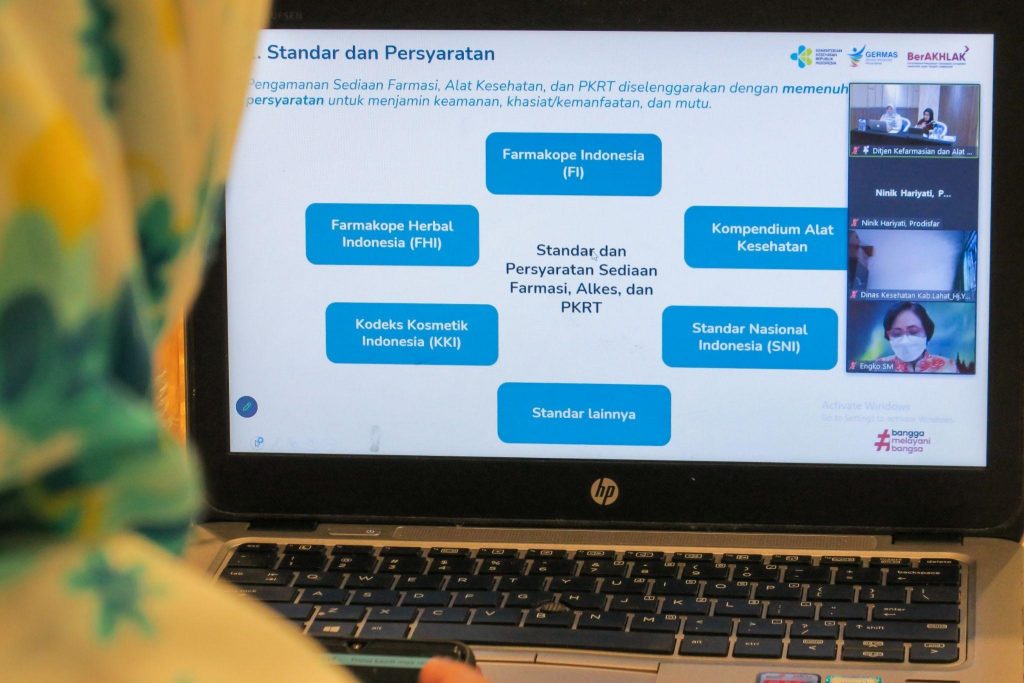
Director of Medical Device Control, Eka Purnamasari through her presentation said that the Safety of Pharmaceutical Preparations, Medical Devices and Household Health Supplies aims to protect the public from hazards caused by the use of pharmaceutical preparations, medical devices, and PKRT that do not meet safety, efficacy / usefulness, and quality requirements.
"Products in circulation must meet the requirements referred to by meeting standards to ensure safety, efficacy / usefulness, and quality. Quality assurance must be guarded by the government and business actors comprehensively from production to circulation, among others through sampling and testing carried out periodically within a certain time intensively," said Eka.
Furthermore, Eka said that production and distribution facilities must meet the applicable rules or regulations. With the advancement of information technology, circulation which includes distribution and delivery can also be done by utilizing an integrated electronic system, namely the National Health Information System (SIKN). The RPP also regulates the importation and exportation of pharmaceutical preparations, medical devices and PKRT, including parties that can import and export and product requirements. For research needs, Research Institutions can import, but are prohibited from distribution. In certain circumstances, imports of pharmaceutical preparations and medical devices that do not have a business license (distribution permit) can be carried out through special channels.

"Pharmaceutical preparations, medical devices and PKRT can be circulated after having a distribution license and fulfilling the marking provisions, can be promoted and advertised in information media by containing objective, complete, and not misleading information and complying with advertising ethics. Meanwhile, prescription drugs and medical devices whose use requires the assistance of medical personnel or health workers may only be promoted and advertised in scientific media for the health profession," Eka said.
The public can participate in realizing protection from hazards caused by the use of pharmaceutical preparations, medical devices, and PKRT that are not appropriate and/or do not meet standards and/or requirements. The central and regional governments in accordance with their respective duties and authorities will provide guidance and supervision on the fulfillment of requirements for safety, efficacy / usefulness, and quality of pharmaceutical preparations, medical devices and PKRT.The Ministry of Health will continue to gather input and aspirations from the public as widely as possible.
The general public can follow this activity through youtube Ministry of Health RI and can actively participate by providing input and suggestions through the website page. https://partisipasisehat.kemkes.go.id/ during the drafting process of the Government Regulation



 Public Hearing on Draft Derivative Regulations of Health Law Number 17 of 2023
Public Hearing on Draft Derivative Regulations of Health Law Number 17 of 2023















